FP003 Quick Answer
R. L. Snyder, Biometeorology Specialist
Department of Land, Air and Water Resources
University of California
Davis, CA 95616, USA
The dew point temperature is defined as the temperature at which the air becomes saturated with water vapor when the air is cooled by removing sensible heat. It is very important in meteorology because it is directly related to the amount of water vapor in the air and it can be used to determine other variables (e.g., vapor pressure, relative humidity, wet bulb temperature, and vapor pressure deficit) that are often used in agriculture. In addition, the dew point measured during nighttime is often a good first guess for the next morning’s minimum temperature. Consequently, it is extremely important for freeze protection of crops. The definition of the dew point temperature is given in the figure below.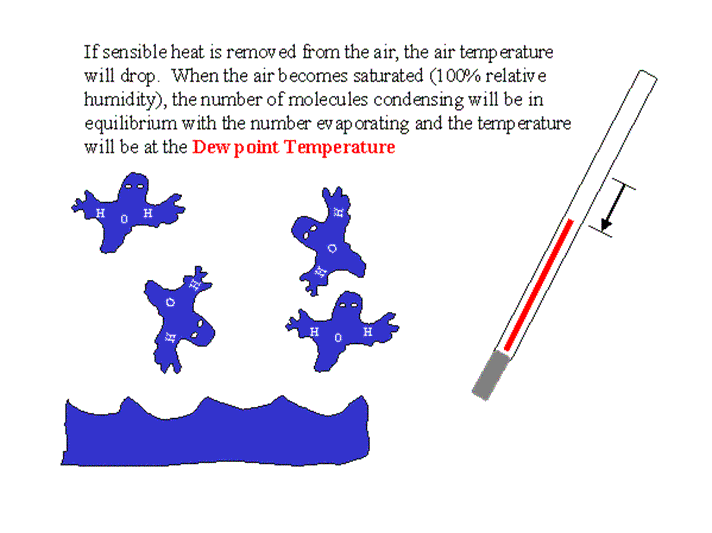
Nighttime Energy Balance and Inversions
During nighttime, there is a net loss of long wave radiation from the surface to the sky. This causes the surface to cool and sensible heat, which is measured with a thermometer, is convected downward from the air to the surface to partially replace the heat loss. However, the surface cools faster than the air above and this usually leads to an inversion (i.e., the temperature increases with height). Also, heat is conducted upward through the soil to partially replace the heat lost to radiation. The net radiation is relatively constant during the night, but the volume of air supplying heat to the surface increases during the night and so the net rate of heat loss of air near the surface decreases with time during the night. Similarly the net rate of heat loss of the soil layer near the surface decreases with time because heat is being transferred from deeper in the soil. As a result, the air temperature drops fast immediately after sunset, but the rate of temperature drop slows near dawn.
Sensible and Latent Heat
Gaseous water molecules consist of one oxygen and two hydrogen atoms. When in a liquid state, the water molecules form strong hydrogen bonds between the molecules. In order to evaporate water, heat must be supplied to break the hydrogen bonds and allow individual molecules to break off as a gas. This is what happens when water is boiled. The heat supplied by your stove breaks hydrogen bonds and the liquid water is rapidly evaporated. Therefore, heat must be supplied to break hydrogen bonds and evaporate water. If air is the source of the heat (called sensible heat), then the air temperature will drop and the energy is stored as chemical energy in the water molecules (called latent heat). When water vapor condenses into liquid water, the hydrogen bonds form again and release latent heat, which increases the sensible heat and causes the air temperature to rise. This exchange between latent and sensible heat is one of the most important factors controlling our climate and environment. Much of the heat transfer on a global scale results from water evaporating at the surface near the equator and condensing into clouds and moving poleward to redistribute the energy. The main point is that sensible heat is removed from the air and the temperature drops when evaporation is occurring and latent heat is converted to sensible heat and the temperature rises when condensation occurs.
Dew Formation
When the surface temperature reaches the dew point temperature, dew will form. Note that dew does not fall from the sky. Water vapor, like other gases, moves at sonic speeds and continually strikes the surface. When the surface temperature is at the dew point, more water molecules will condense onto the surface than evaporate from the surface. Hence, dew forms when the number of water molecules striking the surface and forming hydrogen bonds with other water molecules is bigger than the number of molecules breaking hydrogen bonds and separating off as a gas.
The Ice-Water Can Method to Measure Dew Point
Based on the definition, a simple method to measure the dew point temperature involves cooling a surface until water vapor begins to condense on the surface. This is the principle used in a chilled mirror hygrometer, which is used to measure the dew point. Unfortunately, a chilled mirror uses complicated electronics to measure the dew point and; therefore, it is expensive. A simple, inexpensive method involves using a shinny can, a thermometer, and ice water as shown below.
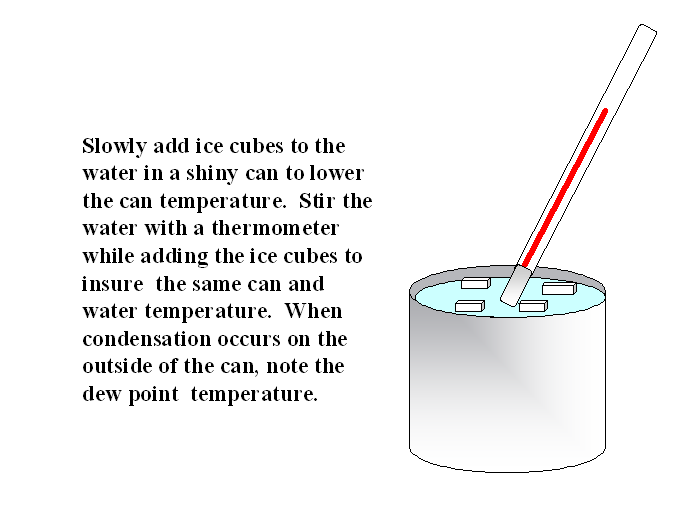
During low dew point, freezing conditions, it is sometimes difficult to get the can cold enough for condensation to occur. Adding salt to the liquid ice mixture will help to melt the ice and cool the water. When the temperature is well below freezing, sometimes frost rather than dew will form on the outside of the can. When this occurs, you have measured the “frost point” rather than the dew point temperature. For the same vapor pressure, the frost point will be slightly higher than the dew point temperature. However, for most agricultural operations, there is little difference and they can be used interchangeably.
Conversions
The equations for converting humidity expressions are typically given using the metric system and degrees Celsius. To convert from degrees Fahrenheit (oF) to degrees Celsius (oC), use the following: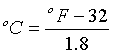
To convert from the dew point temperature (Td) in oC to other expressions for humidity, first calculate the vapor pressure (e).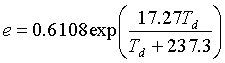 (kPa)
(kPa)
If you measured the frost point temperature (Tf) in oC, then use the following: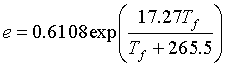 (kPa)
(kPa)
To calculate the saturation vapor pressure (es) at air temperature (T) in oC, use the following:>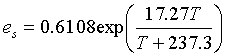 (kPa)
(kPa)
Relative humidity (RH) is calculated as: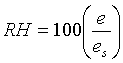 (%)
(%)
Vapor pressure deficit (VPD) is calculated as:![]() (kPa)
(kPa)
Last updated June 22, 2000